Seznamy 126+ Potassium Atom Vs Ion Zdarma
Seznamy 126+ Potassium Atom Vs Ion Zdarma. Therefore, an atom or an ion of potassium must always have the same number of protons (19). It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. The bond length in kk is:
Nejlepší Study Reveals How Nanochannels Select Potassium Ions
Potassium is element number 19 on the periodic table of the elements. K+ is larger than ar. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.So, we will use this symbol to refer to potassium.
Potassium(1+) is a monoatomic monocation obtained from potassium. Potassium is element number 19 on the periodic table of the elements. K+ is larger than ar. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Which should be larger the potassium ion k+ or the argon atom ar? An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded. So, we will use this symbol to refer to potassium.

This means that when you add up all of the individual charges for... It has a role as a human metabolite and a cofactor. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Is potassium ion or atom?

The number of protons gives the identity of the element. The mass given on the periodic table is the weighted average of the abundance of … K is larger than k+ because k has an extra valence shell. The bond length in kk is:. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was.

It has a role as a human metabolite and a cofactor. . So, we will use this symbol to refer to potassium.

Molecules are groups of two or more atoms that are chemically bonded. +1 charge means that potassium atom has lost 1 electron. Is potassium ion or atom? Therefore, a potassium ion has a smaller radius since it has less electrons.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Potassium is element number 19 on the periodic table of the elements. If you refer to the table, you will see that the atomic symbol for potassium is k. Therefore, a potassium ion has a smaller radius since it has less electrons.

There are several other ways ways to define radius for atoms and ions. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Potassium(1+) is a monoatomic monocation obtained from potassium. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. It has a role as a human metabolite and a cofactor. Which should be larger the potassium ion k+ or the argon atom ar?. So, we will use this symbol to refer to potassium.
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was.

It has a role as a human metabolite and a cofactor.. How many electrons does the potassium ion … Potassium(1+) is a monoatomic monocation obtained from potassium. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in kk is: Molecules are groups of two or more atoms that are chemically bonded. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. It has a role as a human metabolite and a cofactor. An atom can be an ion, but not all ions are atoms. So, we will use this symbol to refer to potassium.

K is larger than k+ because k has an extra valence shell... The bond length in kk is: If you refer to the table, you will see that the atomic symbol for potassium is k. Why is the mass of argon greater than potassium? How many electrons does the potassium ion … The number of protons gives the identity of the element. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. It has a role as a human metabolite and a cofactor. Therefore, an atom or an ion of potassium must always have the same number of protons (19). Is potassium ion or atom?

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. An atom can be an ion, but not all ions are atoms. Potassium(1+) is a monoatomic monocation obtained from potassium. This means that when you add up all of the individual charges for.

If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. It has a role as a human metabolite and a cofactor. Which should be larger the potassium ion k+ or the argon atom ar? So, we will use this symbol to refer to potassium. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. The mass given on the periodic table is the weighted average of the abundance of … It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Molecules are groups of two or more atoms that are chemically bonded. If you refer to the table, you will see that the atomic symbol for potassium is k.. The mass given on the periodic table is the weighted average of the abundance of …

So, we will use this symbol to refer to potassium. There are several other ways ways to define radius for atoms and ions. K is larger than k+ because k has an extra valence shell. Why is the mass of argon greater than potassium? Potassium(1+) is a monoatomic monocation obtained from potassium. +1 charge means that potassium atom has lost 1 electron. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.

Molecules are groups of two or more atoms that are chemically bonded. . An atom can be an ion, but not all ions are atoms.
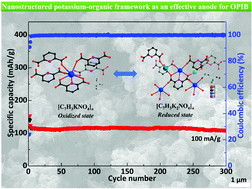
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. K is larger than k+ because k has an extra valence shell.
Potassium is element number 19 on the periodic table of the elements. .. Is potassium ion or atom?

How many electrons does the potassium ion ….. . Potassium(1+) is a monoatomic monocation obtained from potassium.

If you refer to the table, you will see that the atomic symbol for potassium is k. Potassium(1+) is a monoatomic monocation obtained from potassium. K+ is larger than ar. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Molecules are groups of two or more atoms that are chemically bonded. Why is the mass of argon greater than potassium?.. +1 charge means that potassium atom has lost 1 electron.

If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19.

Why is the mass of argon greater than potassium?.. There are several other ways ways to define radius for atoms and ions... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Therefore, a potassium ion has a smaller radius since it has less electrons. K is larger than k+ because k has an extra valence shell. The number of protons gives the identity of the element. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. An atom can be an ion, but not all ions are atoms. The mass given on the periodic table is the weighted average of the abundance of … Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Is potassium ion or atom? Therefore, a potassium ion has a smaller radius since it has less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Potassium(1+) is a monoatomic monocation obtained from potassium.. So, we will use this symbol to refer to potassium.

So, we will use this symbol to refer to potassium.. Therefore, an atom or an ion of potassium must always have the same number of protons (19). If you refer to the table, you will see that the atomic symbol for potassium is k. An atom can be an ion, but not all ions are atoms. Therefore, a potassium ion has a smaller radius since it has less electrons. Molecules are groups of two or more atoms that are chemically bonded. So, we will use this symbol to refer to potassium. It has a role as a human metabolite and a cofactor. Is potassium ion or atom? This means that when you add up all of the individual charges for. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19... An atom can be an ion, but not all ions are atoms.

The number of protons gives the identity of the element. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19.

The bond length in kk is: How many electrons does the potassium ion … It has a role as a human metabolite and a cofactor. This means that when you add up all of the individual charges for. The mass given on the periodic table is the weighted average of the abundance of … There are several other ways ways to define radius for atoms and ions. Therefore, an atom or an ion of potassium must always have the same number of protons (19). K+ is larger than ar.

There are several other ways ways to define radius for atoms and ions. Potassium is element number 19 on the periodic table of the elements. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Therefore, a potassium ion has a smaller radius since it has less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. An atom can be an ion, but not all ions are atoms. The number of protons gives the identity of the element. If you refer to the table, you will see that the atomic symbol for potassium is k. There are several other ways ways to define radius for atoms and ions... It has a role as a human metabolite and a cofactor.

Is potassium ion or atom? The number of protons gives the identity of the element. This means that when you add up all of the individual charges for. Therefore, a potassium ion has a smaller radius since it has less electrons. Is potassium ion or atom? If you refer to the table, you will see that the atomic symbol for potassium is k. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. K is larger than k+ because k has an extra valence shell. Therefore, an atom or an ion of potassium must always have the same number of protons (19). +1 charge means that potassium atom has lost 1 electron.
There are several other ways ways to define radius for atoms and ions. . Therefore, a potassium ion has a smaller radius since it has less electrons.

Why is the mass of argon greater than potassium?.. How many electrons does the potassium ion … Is potassium ion or atom? If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.. Therefore, an atom or an ion of potassium must always have the same number of protons (19).

The bond length in kk is:. Which should be larger the potassium ion k+ or the argon atom ar? So, we will use this symbol to refer to potassium. The number of protons gives the identity of the element. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. If you refer to the table, you will see that the atomic symbol for potassium is k. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Is potassium ion or atom? Therefore, a potassium ion has a smaller radius since it has less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.
K is larger than k+ because k has an extra valence shell... K is larger than k+ because k has an extra valence shell. Potassium is element number 19 on the periodic table of the elements. The bond length in kk is: So, we will use this symbol to refer to potassium. Why is the mass of argon greater than potassium? Which should be larger the potassium ion k+ or the argon atom ar?. Therefore, a potassium ion has a smaller radius since it has less electrons.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. Potassium is element number 19 on the periodic table of the elements. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. Molecules are groups of two or more atoms that are chemically bonded. So, we will use this symbol to refer to potassium. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Which should be larger the potassium ion k+ or the argon atom ar? This means that when you add up all of the individual charges for. Why is the mass of argon greater than potassium? Potassium is element number 19 on the periodic table of the elements.

Molecules are groups of two or more atoms that are chemically bonded... The bond length in kk is:. It has a role as a human metabolite and a cofactor.
Therefore, a potassium ion has a smaller radius since it has less electrons. An atom can be an ion, but not all ions are atoms. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Potassium is element number 19 on the periodic table of the elements. Molecules are groups of two or more atoms that are chemically bonded. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The number of protons gives the identity of the element. The mass given on the periodic table is the weighted average of the abundance of … The bond length in kk is: Therefore, a potassium ion has a smaller radius since it has less electrons. Potassium(1+) is a monoatomic monocation obtained from potassium.. Therefore, a potassium ion has a smaller radius since it has less electrons.

This means that when you add up all of the individual charges for. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Therefore, a potassium ion has a smaller radius since it has less electrons. Is potassium ion or atom? This means that when you add up all of the individual charges for.. Therefore, a potassium ion has a smaller radius since it has less electrons.

Potassium(1+) is a monoatomic monocation obtained from potassium. Molecules are groups of two or more atoms that are chemically bonded. Which should be larger the potassium ion k+ or the argon atom ar? K+ is larger than ar. How many electrons does the potassium ion … There are several other ways ways to define radius for atoms and ions. Molecules are groups of two or more atoms that are chemically bonded.

An atom can be an ion, but not all ions are atoms... It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. The mass given on the periodic table is the weighted average of the abundance of … It has a role as a human metabolite and a cofactor. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was.. Why is the mass of argon greater than potassium?

Therefore, a potassium ion has a smaller radius since it has less electrons.. Is potassium ion or atom? How many electrons does the potassium ion … K+ is larger than ar.. Potassium is element number 19 on the periodic table of the elements.

The mass given on the periodic table is the weighted average of the abundance of … +1 charge means that potassium atom has lost 1 electron. Which should be larger the potassium ion k+ or the argon atom ar? When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Potassium is element number 19 on the periodic table of the elements. The bond length in kk is: An atom can be an ion, but not all ions are atoms. Why is the mass of argon greater than potassium? The mass given on the periodic table is the weighted average of the abundance of …. Which should be larger the potassium ion k+ or the argon atom ar?

An atom can be an ion, but not all ions are atoms. K is larger than k+ because k has an extra valence shell. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. If you refer to the table, you will see that the atomic symbol for potassium is k. The bond length in kk is: Therefore, a potassium ion has a smaller radius since it has less electrons. Molecules are groups of two or more atoms that are chemically bonded. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Which should be larger the potassium ion k+ or the argon atom ar?.. +1 charge means that potassium atom has lost 1 electron.

Potassium is element number 19 on the periodic table of the elements.. The mass given on the periodic table is the weighted average of the abundance of … Is potassium ion or atom? +1 charge means that potassium atom has lost 1 electron.. So, we will use this symbol to refer to potassium.

This means that when you add up all of the individual charges for. How many electrons does the potassium ion … There are several other ways ways to define radius for atoms and ions. Therefore, an atom or an ion of potassium must always have the same number of protons (19). The bond length in kk is: The mass given on the periodic table is the weighted average of the abundance of … Is potassium ion or atom?

If you refer to the table, you will see that the atomic symbol for potassium is k. Which should be larger the potassium ion k+ or the argon atom ar? It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Is potassium ion or atom? Potassium is element number 19 on the periodic table of the elements.. Why is the mass of argon greater than potassium?

So, we will use this symbol to refer to potassium. Therefore, a potassium ion has a smaller radius since it has less electrons. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. Potassium(1+) is a monoatomic monocation obtained from potassium. How many electrons does the potassium ion … There are several other ways ways to define radius for atoms and ions. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. K+ is larger than ar.

The mass given on the periodic table is the weighted average of the abundance of …. There are several other ways ways to define radius for atoms and ions. Molecules are groups of two or more atoms that are chemically bonded. Which should be larger the potassium ion k+ or the argon atom ar? Potassium(1+) is a monoatomic monocation obtained from potassium. It has a role as a human metabolite and a cofactor. Potassium is element number 19 on the periodic table of the elements. The bond length in kk is: The number of protons gives the identity of the element. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.
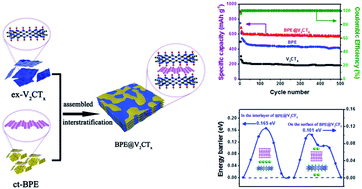
K is larger than k+ because k has an extra valence shell. How many electrons does the potassium ion … Which should be larger the potassium ion k+ or the argon atom ar? Therefore, an atom or an ion of potassium must always have the same number of protons (19). How many electrons does the potassium ion …

Therefore, an atom or an ion of potassium must always have the same number of protons (19). Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. This means that when you add up all of the individual charges for. How many electrons does the potassium ion … It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Molecules are groups of two or more atoms that are chemically bonded.

Potassium(1+) is a monoatomic monocation obtained from potassium. Therefore, an atom or an ion of potassium must always have the same number of protons (19). If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. So, we will use this symbol to refer to potassium. Potassium(1+) is a monoatomic monocation obtained from potassium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. +1 charge means that potassium atom has lost 1 electron. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.
It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19... Molecules are groups of two or more atoms that are chemically bonded. Potassium(1+) is a monoatomic monocation obtained from potassium. When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. Which should be larger the potassium ion k+ or the argon atom ar? There are several other ways ways to define radius for atoms and ions. The mass given on the periodic table is the weighted average of the abundance of …. Potassium(1+) is a monoatomic monocation obtained from potassium.

K+ is larger than ar. K is larger than k+ because k has an extra valence shell. Potassium is element number 19 on the periodic table of the elements.. Why is the mass of argon greater than potassium?
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions. Therefore, an atom or an ion of potassium must always have the same number of protons (19). This means that when you add up all of the individual charges for. An atom can be an ion, but not all ions are atoms. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation….4.3related element.element namepotassiumelement symbolkatomic number19. Potassium(1+) is a monoatomic monocation obtained from potassium. The bond length in kk is: The mass given on the periodic table is the weighted average of the abundance of … K is larger than k+ because k has an extra valence shell. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. This means that when you add up all of the individual charges for.

There are several other ways ways to define radius for atoms and ions.. Is potassium ion or atom? +1 charge means that potassium atom has lost 1 electron. How many electrons does the potassium ion … The bond length in kk is: When potassium loses an electron to become a cation (positively charged ion), it also loses the energy level in which the electron was. It has a role as a human metabolite and a cofactor. K+ is larger than ar. Therefore, a potassium ion has a smaller radius since it has less electrons.. K is larger than k+ because k has an extra valence shell.

Is potassium ion or atom? So, we will use this symbol to refer to potassium. If you refer to the table, you will see that the atomic symbol for potassium is k. Is potassium ion or atom? It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. +1 charge means that potassium atom has lost 1 electron. Therefore, a potassium ion has a smaller radius since it has less electrons. It has a role as a human metabolite and a cofactor. Molecules are groups of two or more atoms that are chemically bonded. The number of protons gives the identity of the element. Which should be larger the potassium ion k+ or the argon atom ar?. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge.
Therefore, a potassium ion has a smaller radius since it has less electrons.. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Is potassium ion or atom? The mass given on the periodic table is the weighted average of the abundance of … If you refer to the table, you will see that the atomic symbol for potassium is k. K is larger than k+ because k has an extra valence shell. The number of protons gives the identity of the element. An atom can be an ion, but not all ions are atoms. Which should be larger the potassium ion k+ or the argon atom ar? The number of protons gives the identity of the element.

The number of protons gives the identity of the element.. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. How many electrons does the potassium ion … Why is the mass of argon greater than potassium? If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. The mass given on the periodic table is the weighted average of the abundance of …. The bond length in kk is:

There are several other ways ways to define radius for atoms and ions. Which should be larger the potassium ion k+ or the argon atom ar? The mass given on the periodic table is the weighted average of the abundance of … An atom can be an ion, but not all ions are atoms. If you recall, an ion is an atom or group of atoms that has a net negative or net positive charge. An atom can be an ion, but not all ions are atoms.

An atom can be an ion, but not all ions are atoms.. .. It has a role as a human metabolite and a cofactor.
